Costello Eye Physicians and Surgeons offers minimally invasive glaucoma surgery to our patients that are candidates and is the leader of MIGS surgery in the Mohawk Valley. They are the first doctors to perform many of the the MIGS procedures in the region. The Hydrus micro-stent is an exciting MIGS procedure for patients with mild and moderate open angle glaucoma undergoing simultaneous cataract surgery.
Hydrus received FDA approval in 2018 through the HORIZON trial, the largest MIGS study conducted to date, where 556 mild to moderate glaucoma patients requiring cataract surgery underwent the procedure with either the Hydrus Microstent or cataract surgery alone. The results were staggering with the Hydrus group experiencing the largest improvement over a control group shown in a MIGS pivotal trial to date. When studied 24 months after the procedure, 77.2 percent of the Hydrus patients achieved a statistically significant decrease in unmedicated interocular pressure with a mean reduction of 7.5 mmHg, compared to the 57.8 percent of control patients showing a similar rate of decrease. These results, along with additional clinical data, are encouraging for the safety and two-year efficacy of the device.
Why the Hydrus Microstent?
In addition to the HORIZON trial, the Hydrus Microstent has been examined in various national and international studies with both stand-alone glaucoma procedures and cataract-glaucoma procedures. As of August, 2019, over 4,000 procedures using the Hydrus Microstent have been performed around the world with patients showing a wide range of glaucoma symptoms.
The Hydrus Microstent is approximately the size of an eyelash – small enough that it is typically never felt or seen by patients. Its design allows for continued dilation of and maximum aqueous flow through Schlemm’s canal – reducing intraocular pressure for patients with mild to moderate primary open-angle glaucoma.
Interested in a Minimally Invasive Approach for Glaucoma Relief?
Lowered eye pressure achieved via the Hydrus Microstent can assist in managing glaucoma symptoms for up to two years per device. Costello Eye Physicians and Surgeons are dedicated to providing the most advanced and minimally invasive glaucoma surgery to our patients. Contact us to see if you are a candidate for the procedures.
Background
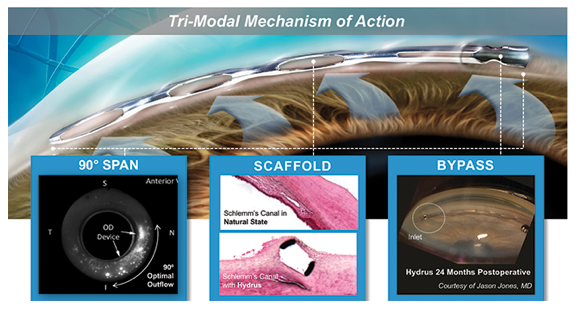
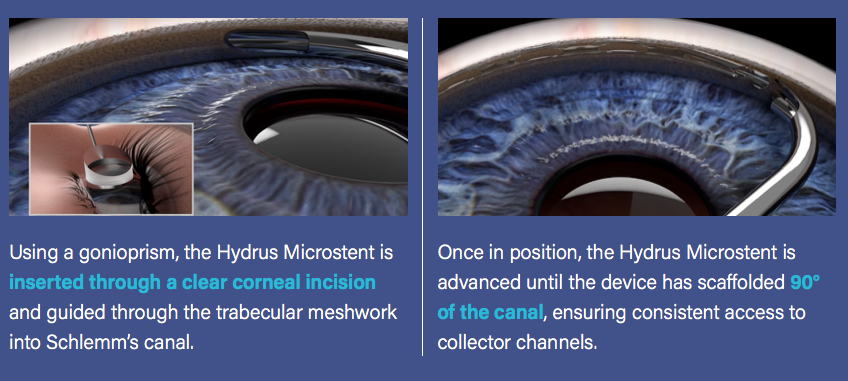
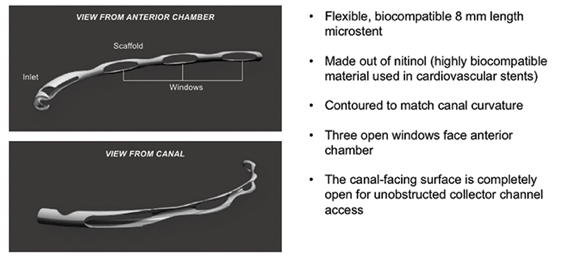
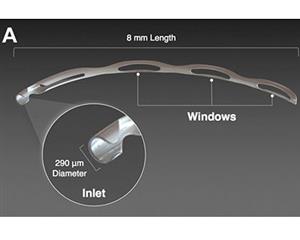
The Hydrus Microstent (Ivantis, Inc, Irvine, CA, USA) is an approximately 8 mm long, curved device that comprises alternating spines for structural support and windows for aqueous outflow (Figure 1). Because of its scaffold design, the microstent occupies the Schlemm’s canal, but does not block the collector channel ostia in the posterior portion of Schlemm’s canal. The microstent is made of nitinol, a nickel-titanium alloy that possesses super-elastic properties, allowing for return to its original shape after deformation. Once inserted, the microstent can dilate Schlemm’s canal by four to five times the natural width of the canal, countering the collapse of Schlemm’s canal induced by elevated IOP
Indications
The Hydrus Microstent was approved by the FDA in 2018 for use in conjunction with cataract surgery to reduce intraocular pressure in adult patients with mild to moderate primary open-angle glaucoma.
Contraindications
- Angle closure glaucoma
- Traumatic glaucoma
- Malignant glaucoma
- Uveitic glaucoma
- Neovascular glaucoma
- Congenital anomalies of the anterior chamber angle
Outcomes
Hydrus versus selective laser trabeculoplasty (SLT)
In the sole study comparing Hydrus versus SLT at the time of this writing, Fea et al. studied the efficacy of standalone Hydrus device implantation compared to SLT in a 12-month, prospective, non-randomized, interventional case series of 56 patients with uncontrolled mild to moderate POAG [5]. In total, 25 eyes underwent SLT, while 31 received Hydrus implantation. There were no statistically significant differences in baseline demographic characteristics, intraocular pressure, or mean number of medications between groups. Washout of all glaucoma medications took place after the procedures. Both groups saw significant decreases in IOP compared to baseline, though the between-group differences in both mean and percentage IOP reduction were statistically insignificant at all follow-up visits. At 12 months, 90% of Hydrus patients and 88% of SLT patients achieved IOP reduction greater than 20%. Despite essentially equivalent outcomes in IOP among the treatment groups, there was a statistically significant difference in drug reduction at 12 months between groups (-1.4 ± 0.97 in Hydrus patients vs.-0.5 ± 1.05 in SLT patients), and only Hydrus patients achieved a significant within-group drug reduction from baseline at 12 months. Furthermore, 47% of Hydrus patients were medication-free at 12 months, versus only 4% of SLT patients.
Hydrus vs iStent
The COMPARE study was the first to compare two different MIGS devices in standalone surgery, rather than in combination with phacoemulsification. This was a prospective, multicenter, randomized controlled single-masked clinical trial in which 152 eyes with open-angle glaucoma (primary and select secondary diagnoses, including pseudoexfoliative and pigmentary glaucoma) were randomized 1:1 to either receive one Hydrus device or two iStents, with medication washout performed prior to surgery [6]. For all endpoints at 12 months, Hydrus outcomes were superior to those of the two-iStent group, even on subgroup analysis. Baseline diurnal IOP (DIOP) was similar between the two groups, both pre- and post-medication washout, as were number of glaucoma medications used (2.5, 2.7). Patients in the Hydrus group had a greater reduction in mean IOP than those in the iStent group (-1.7 vs. -1.0 mm Hg), though this was not statistically significant. However, the Hydrus group achieved a greater decrease in number of medications than the iStent group (-1.6, -1.0), and had a higher proportion of patients who were medication-free at 12 months compared to the iStent group (46.6% vs. 24.0%). Among all patients at 12 months (including those who were not medication-free), mean IOP was lower (17.3, 19.2), change in IOP was greater (-8.2, -5.1), and more patients in the Hydrus group achieved >20% IOP reduction from the washed out baseline DIOP (39.7%, 13.3%). Conversely, there were fewer Hydrus patients that needed 3 or more medications at 12 months than iStent patients (8.2%, 29.3%).
Of note, among the first 40 subjects to reach 12 months’ follow-up, approximately 20% of eyes in the 2 iStent group had IOP elevation refractory to maximum tolerated medical therapy. Investigators were reluctant to perform a medication washout in these eyes at this time, causing a deviation from the original study design in which the washout requirement was waived for the 64th patient to reach the 12-month follow-up and all patients after that. This protocol change eliminated the ability to directly compare device-related IOP reductions and limits ability to reach definitive conclusions about the efficacy of the two devices.
Hydrus with cataract surgery vs cataract surgery alone
In several studies, concurrent Hydrus device implantation with cataract surgery (CS) appears to be superior in lowering IOP and reducing the number of glaucoma medications than CS alone, and equivalently safe. In the HYDRUS II study, a 2-year prospective, multicenter, randomized controlled single-masked clinical trial, Pfeiffer et al. analyzed 100 eyes with open-angle glaucoma, who all underwent washout of ocular hypotensives prior to surgery [8]. Patients were randomized 1:1 to undergo CS with the microstent or CS alone. Washout of hypotensive medications was repeated at 12 and 24 months. After accounting for study dropout and need for additional incisional glaucoma surgery, 90 patients remained in the study after 24 months. Washed out mean DIOP in the Hydrus plus CS group was significantly lower at 24 months compared with the CS group (16.9 +/- 3.3 mmHg vs. 19.2 +/- 4.7 mmHg; P = 0.0093). The proportion of patients with a decrease in washed out DIOP of 20% or more compared to baseline was significantly higher in the Hydrus plus CS group at 24 months compared with the CS group (80% vs. 46%; P = 0.0008). More patients in the Hydrus plus CS group were medication-free at 24 months than patients in the CS group (73% vs. 38%; P = 0.0008).
In a 2-year retrospective, multicenter case-series, Fea et al. analyzed the effectiveness of the Hydrus microstent combined with cataract surgery in 92 eyes, including 6 that had undergone prior glaucoma surgery (4 trabeculectomies, 1 tube shunt, 1 cyclodiode laser) [9]. On subgroup analysis, 42 eyes fell in group 1 (IOP ≤ 18 mmHg), while the other 50 fell in group 2 (IOP ≥ 19 mmHg); baseline characteristics aside from IOP and glaucoma medication usage (1.86 +/- 0.9, 2.40 +/- 1.1) were similar between the groups. Mean follow-up among all patients was 20.0 +/- 7.3 months, with 67 patients ultimately completing the 2-year visit. The mean baseline IOP was 19.4 +/- 4.4 mm Hg, which decreased to 15.6 +/- 2.9 mm Hg at 6 months and was sustained at 2 years (P < .0001). The amount of IOP reduction was positively correlated with baseline IOP (R2 = 0.721). Mean number of medications decreased significantly from 2.1 +/- 1.0 to 0.7 +/- 1.0 at 2 years (P < .0001). At the final follow-up visit, 64% of patients were medication free. On subgroup analysis, in group 1, the mean IOP of 15.7 mm Hg did not change significantly throughout the follow-up period (P > .05). However, the number of medications was statistically significantly reduced to a mean of 0.2 +/- 0.5 at 1 year and 0.5 +/- 0.7 at 2 years (P < .0001). Moreover, 69% of patients were off medication at the final study visit. In group 2, mean IOP decreased significantly from the baseline of 22.6 to 16.0 +/- 3.2 mm Hg at 1 year and 15.7 +/- 2.3 mm Hg at 2 years (P < .0001). The number of medications used also significantly decreased to a mean of 1.0 +/- 1.2 at 2 years. Moreover, 60% of patients were off medications at the final foll
ow-up. Based on success criterion 1 (IOP </=18), the Kaplan-Meier survival rate was 70% of patients at 1 year and 52% at 2 years. Based on success criterion 2 (IOP </=15), the survival was reduced to 36% at 1 year and 25% at 2 years. One patient required a trabeculectomy (at 18 months) during the follow-up. In the HORIZON study, a 2-year prospective, multicenter, randomized controlled single-masked clinical trial, Samuelson et al. analyzed 556 eyes [4]. Based on randomization that was stratified by site and by history of SLT, 369 of the eyes were assigned to the Hydrus microstent (HMS) group, and the other 187 were to receive cataract surgery alone with no microstent (NMS). Medication washout was performed at 12 and 24 months. For all endpoints at 24 months, HMS outcomes were superior to NMS, even on subgroup analysis. 77.3% of HMS patients achieved a reduction of unmedicated mean DIOP of >20%, versus only 57.8% of NMS patients. HMS eyes also saw a greater mean reduction of unmedicated mean DIOP than NMS eyes (-7.6 vs. -5.3 mm Hg). While patients in both groups were using an average of 1.7 glaucoma medications prior to the study, HMS patients were only using 0.3 on average, while NMS patients were taking 0.7. Furthermore, the proportion of patients who were medication-free at 24 months was higher in the HMS group (78 percent) than in the NMS group (48 percent).
Complications
Reported intraoperative complications in clinical studies of the Hydrus have been uncommon and typically involve transient hyphema or malpositioning of the device. Hyphema has been noted at a rate of about 1.1-2%, and usually resolves within one week post-operatively [4,5,8]. Malpositioning of the stent within the iris root or outside of Schlemm’s canal, which may require intraoperative scaffold repositioning, has also been reported [5]. Other noted complications include cyclodialysis cleft, iridodialysis, corneal abrasions, and Descemet membrane detachment.
Conclusions
In clinical trials, the Hydrus Microstent has been shown to serve as a useful adjunct to phacoemulsification for reduction of IOP and need for ocular hypotensive medication in mild to moderate open-angle glaucoma. From the available data, outcomes for the Hydrus as a standalone procedure also seem to be at least noninferior to those of selective laser trabeculoplasty and the iStent, with a greater proportion of patients found to have controlled IOP without medications. Further follow-up is needed to elucidate adverse effects in the long term, but its overall favorable safety profile and ability to spare conjunctiva should there be need for future incisional glaucoma surgery bode well for the addition of the Hydrus to the surgical arsenal of glaucoma management.
Hydrus Microstent
- FDA approved August 2018
- Implantable, flexible metal (nitinol) tube with windows (open-back stent)
- Adults with mild-moderate POAG
- Only approved with PHACO surgery at present
- Reduction of IOP with cataract surgery >20% in 77.2% of patients vs 57.8% with cataract surgery alone
- Contraindicated:
- Angle closure
- NVG
- Traumatic glaucoma
Hydrus vs 2 Istents – comparison trial
- COMPARE trial
- Patients with mild to moderate glaucoma taking 2 or more medications
- Prospective multi-center trial
- 1:1 assignment to one or other procedure
- Data released April 2018
- Hydrus FDA approval August 2018
- Hydrus statistically superior at lowering the medication count
- At 12 months
- 46% of patients required to take no medication with Hydrus
- 24% of patients no medication with 2 IStents





