The doctors at Costello Eye Physicians and Surgeons are the first physicians in the Mohawk Valley Region to insert XEN 45 Gel Implant.
This procedure can be used in Moderate and Severe open angle glaucoma patients that have failed to responded to medications, laser or other interventions or are no longer controlled on medical therapy.

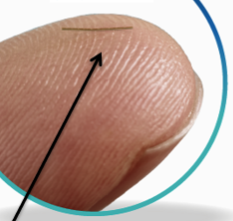
This highly magnified view of the XEN® Gel Stent reveals that it’s a small tube that, when inserted into the eye, becomes soft and flexible. XEN® is designed to help lower eye pressure and was evaluated in a U.S. clinical study that established its safety and effectiveness.
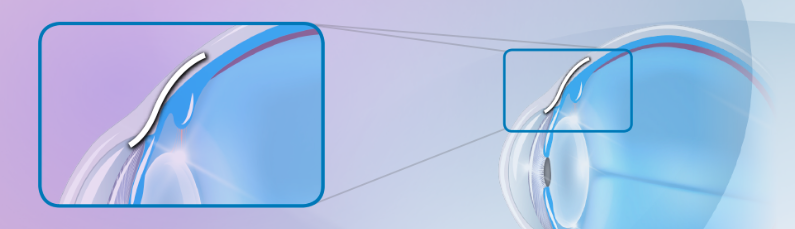
The XEN® Gel Stent creates a small channel in the eye to drain fluid and help lower eye pressure. The XEN® Gel Stent is tiny—about the length of an eyelash—and it’s placed just under the conjunctiva, which is a clear membrane that covers the white of your eye.
You may or may not need to use glaucoma eye drops after the XEN®procedure. Your doctor will determine your need for eye drops after the XEN® procedure.
The XEN® Gel Stent is designed to stay in the eye permanently.
As with all procedures, there can be risks, which your doctor will discuss with you. If you have open-angle glaucoma and your previous surgical treatment has failed and/or medications alone were insufficient, your doctor can help you decide if the XEN® Gel Stent is the right choice for you. Individual results may vary.
Pre-operative:
- Patients are instructed to stop all glaucoma medications on the day of the operation.
Post-operative:
- Patients are seen postoperative day 1 and followed up at the physician discretion.
- Postoperative topical regimen includes topical antibiotic prophylaxis for 4 weeks and topical corticosteroid 4 times each day for a month followed by a slow taper over the second month.
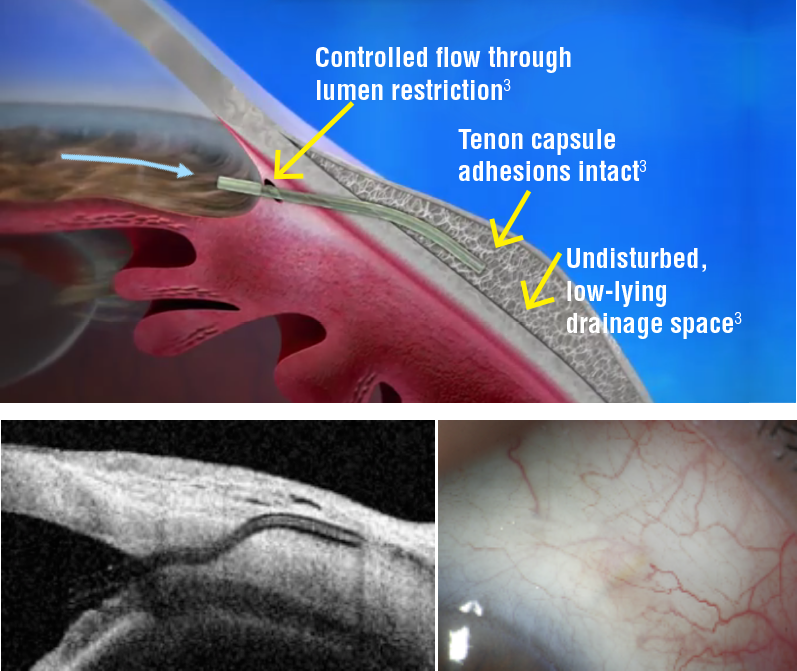
- XEN implant location and subconjunctival bleb morphology require close biomicroscopic follow-up, complemented with UBM (FIG1) or AS-OCT.
Morphologic changes to the developing filtering bleb after surgery may help to predict early treatment failure and guide bleb revision and management. Fea et al. reported that maximal height of the bleb and the total area of cystic hypoehoic spaces were significantly higher in the success group. Increased micro-cysts and loosely arranged connective tissue/low stromal reflectivity are suggestive of new or increased alternative outflow induced by the stent implantation. In the other hand, bleb wall reflectivity was significantly higher in the failure group.
- Needling may be performed in case of IOP increases and is greater than target IOP; there is a flat bleb or fibrotic bleb or the patient is at high risk of bleb failure.
Xen Implant Advantages
Xen Implant has some advantages over traditional surgeries such as trabeculectomy and tube shunt surgeries:
- Soft and flexible when wet;
- Minimally invasive, pre-loaded injector;
- Shorter surgical and recovery times;
- Less side effects: Eg. Prevents chronic hypotony by an intrinsic flow-limiting design
- Keep postoperative options open, allowing physicians to use other IOP-reduction techniques that could be required after surgery
Complications
Reported Intra- and post-operative complications are minor and inherent to the surgical technique. A critical point is the final placement of the XEN device.
Intra-operative complications:
- Misplacement of the XEN Gel Implant at the first attempt;
- Posterior placement of the implant, especially through ciliary body can lead to bleeding and hypotony;
- Subconjunctival or AC bleeding during the implantation.
Late complications:
- Device erosion and exposure of implant (add XEN erosion Fig);
- Implant migration: Dislocation into AC, XEN-iris touch;
- Bleb leak or dehiscence (may be due to thin or ischemic bleb with overfiltration);
- Reported cases of blebitis and persistent hypotony, suprachoroidal bleeding, conjunctival perforation and late seidel.
Efficacy and Safety Profile
The Implant was recently introduced to glaucoma surgeries, thus long-term results are not available yet. Studies with 1-year follow-up have found it to be efficacious in lowering IOP significantly and reducing the number of hypotensive medications used, with minimal complications or serious side effects. Most studies document an IOP reduction of >29% (greater than the reduction demonstrated by isolated phacoemulsification in patients with primary open-angle glaucoma) and a significant reduction in the number of IOP-lowering medications.
In Galal et al. study, 13 eyes with primary open angle glaucoma underwent XEN implantation with subconjunctival MM. Complete success (IOP reduction ≥20% from preoperative baseline at 1 year without any glaucoma medications) was achieved on 41.7% of patients, while qualified success rate (IOP reduction of ≥20% at 1 year with medications) was 66.7%. IOP reduction at 12 months was 16 ± 4 to 12 ± 3 mmHg (p ≤ 0.01) and medication’s reduction at 12 months was 1.9 ± 1 to 0.3 ± 0.49 (p = 0.003).
In a prospective, non-RCT by De Gregoria et al, 41 eyes of 33 patients underwent a XEN Gel Implant procedure combined with cataract surgery. Outcomes of this study show that XEN45 implant is statistically effective in reducing IOP and medications even after 12 months. The complete success rate (≤18 mmHg without medications) after 12 months was achieved in 80.4% (33 of 41 eyes) and a qualified success (≤18 mmHg with medications) in 97.5% (40 of 41 eyes). IOP reduction at 12 months was 22.5 ± 3.7 to 13.1 ± 2.4 mmHg (p < 0.05) and medication’s reduction at 12 months was 2.5 ± 0.9 to 0.4 ± 0.8 (p < 0.05).
No serious complication was observed in studies highlighting the safety of the XEN Gel Implant. There was no report on loss or worsening of visual acuity.
- Scleral tunnel
- Stand alone or combined with phaco
- Patients who have failed previous therapy or procedure
- SLT
- Other MIGS
- Medication failure or side effects
- Soft, permanent, subconjunctival implant that shunts fluid from the anterior chamber to the subconjunctival space
- 6-mm longh
- Width of human hair (45um)
Pivotal Xen trial
- Reduced IOP from a mean medicated baseline of 25.1 (+3.7)mmHg to 15.9 (+5.2)mmHg at 12 months post op
- Allows other IOP lowering methods after implantation`





