One Device. One Incision.
Two combined MIGS procedures.
The OMNI® Glaucoma Treatment System is the only device that combines two well-established micro-invasive glaucoma surgery (MIGS) procedures into one using a single device and single clear corneal incision.
With dual mechanisms of action targeting the trabecular mesh-work and collector channels, OMNI® is designed to address the entire natural trabeculocanalicular outflow pathway to reduce intraocular pressure in adult patients with open-angle glaucoma. Can be used for mild, moderate and severe primary and secondary open angle glaucoma.
Titratable Therapy Facilitating up to 360° of Canal Treatment
- Treats all three points of resistance, the trabecular mesh-work, Schlemm’s Canal and the distal collector channels, to reduce IOP
- Viscodilates up to 360° of Schlemm’s canal
- Unroofs up to 360° of the trabecular mesh-work
Efficient Minimally Invasive Canal Surgery
- Ab-interno approach spares the conjunctiva and sclera of incisions
- Implant free and sutureless
- Stand-alone procedure or combined with cataract surgery in a single OR session
The doctors at Costello Eye Physicians and Surgeons at the first doctors in the Mohawk Valley Region. Our doctors have been performing this procedure since the fall of 2019. Because it is minimally invasive, it has a quick recovery. Most patients see a significant response because of the two mechanisms of action.
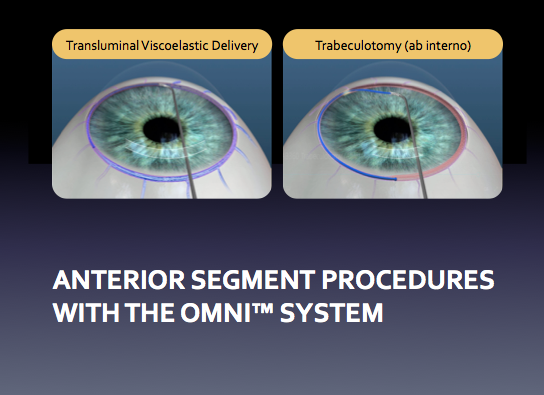
Post operatively, the following instructions are given to the patient. If combined with cataract surgery, some additional cataract procedure drops will be given.

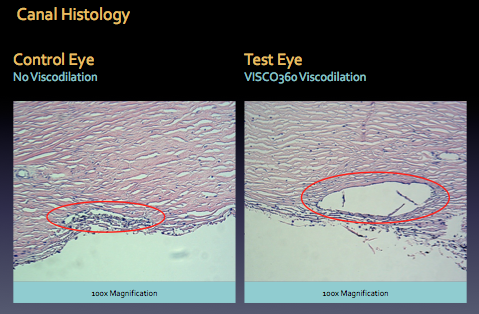
The histology slide below shows Schlemm’s drainage canal in the eye before and after visculocanulodilation surgery.
Results from the OMNI trial studies are shown below
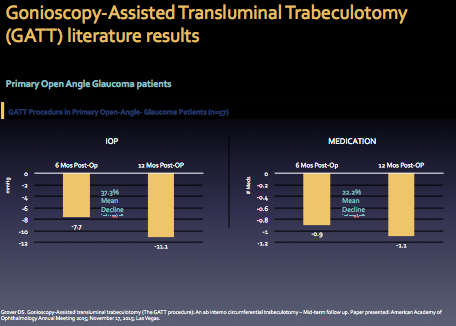

Results from the OMNI trial studies are shown below
MIGS Data for the OMNI Surgical System Presented at European Society of Cataract and Refractive Surgeons
18-month Pilot Study Results from the First OMNI® Cases Performed in Europe Suggest Long-term IOP and Medication Reduction in Mild to Moderate Primary Open Glaucoma
MENLO PARK, Calif., Sept. 18, 2019 /PRNewswire/ — Sight Sciences announced clinical results from the first study of the OMNI® Surgical System in glaucoma patients were presented at the European Society of Cataract and Refractive Surgery (ESCRS). Findings from the pilot study suggest the OMNI® Surgical System, which is dually indicated in Europe for the sequential ab interno procedures of microcatheterization and transluminal viscodilation of Schlemm’s Canal followed by transluminal trabeculotomy, may provide a favorable safety profile and significant long-term reductions in intraocular pressure (IOP) and IOP-lowering medications.
The pilot study included 24 eyes from 19 consecutive patients with mild to moderate primary open-angle glaucoma (POAG) who were treated with the OMNI® Surgical System. The study was conducted at the Ophthalmology Clinic Postgraduate Centre of Medical Education in Warsaw, Poland by Professor Grabska-Liberek, recent President of the Polish Ophthalmology Society.
Key findings include:
- Canal viscodilation followed by trabeculotomy using the OMNI® Surgical System with or without cataract surgery resulted in substantial IOP reduction and IOP-lowering medication reductions at 18 months.
- Mean 40 percent IOP reduction (21.4 mmHg to 12.8 mmHg) at 18 months
- Mean 41 percent medication reduction (2.9 meds to 1.7 meds) at 18 months
- The most common adverse events were IOP-spikes (nine eyes), hyphema (six eyes) and fibrin in the anterior chamber (five cases), all of which resolved in the first week after surgery.
- No eyes required additional surgical intervention to control IOP over the 18-month follow-up period.
If you are having cataract surgery and you have glaucoma, talk to our doctors about MIGS and the Omni Surgical System for glaucoma.
If you are having trouble controlling your eye pressure or would prefer to have laser or a minimally invasive glaucoma procedure over medications or major surgery, talk to our doctors about MIGS and the Omni Surgical System for glaucoma.






